Identification
- Generic Name
- Technetium Tc-99m arcitumomab
- DrugBank Accession Number
- DB00113
- Background
Reduced Fab fragment of the murine IgG1 monoclonal antibody IMMU-4 (also called NP-4) with specificity for carcinoembryonic antigen (CEA) covalently labeled with Technitium 99. The molecule has a molecular weight of ~54,000 Daltons.
- Type
- Biotech
- Groups
- Experimental
- Biologic Classification
- Protein Based Therapies
Monoclonal antibody (mAb) - Protein Structure
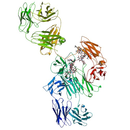
- Protein Chemical Formula
- C6398H9900N1714O1995S54
- Protein Average Weight
- 144482.5 Da
- Sequences
>1clo:Anti-CEA heavy chain 1 EVKLVESGGGLVQPGGSLRLSCATSGFTFTDYYMNWVRQPPGKALEWLGFIGNKANGYTT EYSASVKGRFTISRDKSQSILYLQMNTLRAEDSATYYCTRDRGLRFYFDYWGQGTTLTVS SAKTTPPSVYPLAPGSAAQTNSMVTLGCLVKGYFPEPVTVTWNSGSLSSGVHTFPAVLQS DLYTLSSSVTVPSSPRPSETVTCNVAHPASSTKVDKKIVPRDCPPCKCPAPNLLGGPSVF IFPPKIKDVLMISLSPIVTCVVVDVSEDDPDVQISWFVNNVEVHTAQTQTHREDYNSTLR VVSALPIQHQDWMSGKEFKCKVNNKDLPAPIERTISKPKGSVRAPQVYVLPPPEEEMTKK QVTLTCMVTDFMPEDIYVEWTNNGKTELNYKNTEPVLDSDGSYFMYSKLRVEKKNWVERN SYSCSVVHEGLHNHHTTKSFSR
>1clo: Anti-CEA antigen light chain 1 QTVLSQSPAILSASPGEKVTMTCRASSSVTYIHWYQQKPGSSPKSWIYATSNLASGVPAR FSGSGSGTSYSLTISRVEAEDAATYYCQHWSSKPPTFGGGTKLEIKRADAAPTVSIFPPS SEQLTSGGASVVCFLNNFYPKDINVKWKIDGSERQNGVLNSWTDQDSKDSTYSMSSTLTL TKDEYERHNSYTCEATHKTSTSPIVKSFNRNEC
>1clo:Anti-CEA heavy chain 2 EVKLVESGGGLVQPGGSLRLSCATSGFTFTDYYMNWVRQPPGKALEWLGFIGNKANGYTT EYSASVKGRFTISRDKSQSILYLQMNTLRAEDSATYYCTRDRGLRFYFDYWGQGTTLTVS SAKTTPPSVYPLAPGSAAQTNSMVTLGCLVKGYFPEPVTVTWNSGSLSSGVHTFPAVLQS DLYTLSSSVTVPSSPRPSETVTCNVAHPASSTKVDKKIVPRDCPPCKCPAPNLLGGPSVF IFPPKIKDVLMISLSPIVTCVVVDVSEDDPDVQISWFVNNVEVHTAQTQTHREDYNSTLR VVSALPIQHQDWMSGKEFKCKVNNKDLPAPIERTISKPKGSVRAPQVYVLPPPEEEMTKK QVTLTCMVTDFMPEDIYVEWTNNGKTELNYKNTEPVLDSDGSYFMYSKLRVEKKNWVERN SYSCSVVHEGLHNHHTTKSFSR
>1clo: Anti-CEA antigen light chain 2 QTVLSQSPAILSASPGEKVTMTCRASSSVTYIHWYQQKPGSSPKSWIYATSNLASGVPAR FSGSGSGTSYSLTISRVEAEDAATYYCQHWSSKPPTFGGGTKLEIKRADAAPTVSIFPPS SEQLTSGGASVVCFLNNFYPKDINVKWKIDGSERQNGVLNSWTDQDSKDSTYSMSSTLTL TKDEYERHNSYTCEATHKTSTSPIVKSFNRNEC
Download FASTA Format- Synonyms
- Arcitumomab technetium-99m
- Technetium (99mTc) arcitumomab
- Technetium Tc 99m arcitumomab
- Technetium-99m arcitumomab
Pharmacology
- Indication
For imaging colorectal tumors
 Reduce drug development failure ratesBuild, train, & validate machine-learning modelswith evidence-based and structured datasets.Build, train, & validate predictive machine-learning models with structured datasets.
Reduce drug development failure ratesBuild, train, & validate machine-learning modelswith evidence-based and structured datasets.Build, train, & validate predictive machine-learning models with structured datasets.- Contraindications & Blackbox Warnings
 Prevent Adverse Drug Events TodayTap into our Clinical API for life-saving information on contraindications & blackbox warnings, population restrictions, harmful risks, & more.Avoid life-threatening adverse drug events with our Clinical API
Prevent Adverse Drug Events TodayTap into our Clinical API for life-saving information on contraindications & blackbox warnings, population restrictions, harmful risks, & more.Avoid life-threatening adverse drug events with our Clinical API- Pharmacodynamics
Binds to the carcinoembryonic antigen, which is a cell surface protein generally overexpressed in colon (and other) cancers. The radioactive Tc99, which is covalently attached to the antibody, allows radiodiagnostic detection of CEA expressing cells and tumors
- Mechanism of action
Binds selectively to cell-surface carcinoembryonic antigen (CEA) expressed on colorectal tumors.
Target Actions Organism UCarcinoembryonic antigen-related cell adhesion molecule 1 other/unknownHumans - Absorption
Not Available
- Volume of distribution
Not Available
- Protein binding
Not Available
- Metabolism
Most likely removed by opsonization via the reticuloendothelial system, or by human antimurine antibody production
- Route of elimination
Not Available
- Half-life
Approximately 1 hour
- Clearance
Not Available
- Adverse Effects
 Improve decision support & research outcomesWith structured adverse effects data, including: blackbox warnings, adverse reactions, warning & precautions, & incidence rates. View sample adverse effects data in our new Data Library!Improve decision support & research outcomes with our structured adverse effects data.
Improve decision support & research outcomesWith structured adverse effects data, including: blackbox warnings, adverse reactions, warning & precautions, & incidence rates. View sample adverse effects data in our new Data Library!Improve decision support & research outcomes with our structured adverse effects data.- Toxicity
Not Available
- Pathways
- Not Available
- Pharmacogenomic Effects/ADRs
- Not Available
Interactions
- Drug Interactions
- This information should not be interpreted without the help of a healthcare provider. If you believe you are experiencing an interaction, contact a healthcare provider immediately. The absence of an interaction does not necessarily mean no interactions exist.
Drug Interaction Integrate drug-drug
interactions in your softwareAbciximab The risk or severity of adverse effects can be increased when Abciximab is combined with Technetium Tc-99m arcitumomab. Adalimumab The risk or severity of adverse effects can be increased when Adalimumab is combined with Technetium Tc-99m arcitumomab. Aducanumab The risk or severity of adverse effects can be increased when Technetium Tc-99m arcitumomab is combined with Aducanumab. Alemtuzumab The risk or severity of adverse effects can be increased when Alemtuzumab is combined with Technetium Tc-99m arcitumomab. Alirocumab The risk or severity of adverse effects can be increased when Technetium Tc-99m arcitumomab is combined with Alirocumab. - Food Interactions
- Not Available
Products
 Drug product information from 10+ global regionsOur datasets provide approved product information including:dosage, form, labeller, route of administration, and marketing period.Access drug product information from over 10 global regions.
Drug product information from 10+ global regionsOur datasets provide approved product information including:dosage, form, labeller, route of administration, and marketing period.Access drug product information from over 10 global regions.- International/Other Brands
- CEA-Scan (Immunomedics Inc)
Categories
- ATC Codes
- V09IA06 — Technetium (99mtc) arcitumomab
- Drug Categories
- Chemical TaxonomyProvided by Classyfire
- Description
- Not Available
- Kingdom
- Organic Compounds
- Super Class
- Organic Acids
- Class
- Carboxylic Acids and Derivatives
- Sub Class
- Amino Acids, Peptides, and Analogues
- Direct Parent
- Peptides
- Alternative Parents
- Not Available
- Substituents
- Not Available
- Molecular Framework
- Not Available
- External Descriptors
- Not Available
- Affected organisms
- Humans and other mammals
Chemical Identifiers
- UNII
- 029JF1SCU8
- CAS number
- 154361-49-6
References
- General References
- External Links
- PubChem Substance
- 46507781
- 230435
- ChEMBL
- CHEMBL2108253
- Therapeutic Targets Database
- DAP001303
- PharmGKB
- PA164746540
- Wikipedia
- Arcitumomab
Clinical Trials
Pharmacoeconomics
- Manufacturers
- Not Available
- Packagers
- Immunomedics Inc.
- Dosage Forms
- Not Available
- Prices
- Not Available
- Patents
- Not Available
Properties
- State
- Liquid
- Experimental Properties
Property Value Source melting point (°C) 61 °C (FAB fragment), 71 °C (whole mAb) Vermeer, A.W.P. & Norde, W., Biophys. J. 78:394-404 (2000) hydrophobicity -0.423 Not Available isoelectric point 8.26 Not Available
Targets

- Kind
- Protein
- Organism
- Humans
- Pharmacological action
- Unknown
- Actions
- Other/unknown
- General Function
- Protein homodimerization activity
- Specific Function
- Not Available
- Gene Name
- CEACAM1
- Uniprot ID
- P13688
- Uniprot Name
- Carcinoembryonic antigen-related cell adhesion molecule 1
- Molecular Weight
- 57559.965 Da
References
- Fuster D, Maurel J, Muxi A, Setoain X, Ayuso C, Martin F, Ortega ML, Fuertes S, Pons F: Is there a role for (99m)Tc-anti-CEA monoclonal antibody imaging in the diagnosis of recurrent colorectal carcinoma? Q J Nucl Med. 2003 Jun;47(2):109-15. [Article]
- Hughes K, Pinsky CM, Petrelli NJ, Moffat FL, Patt YZ, Hammershaimb L, Goldenberg DM: Use of carcinoembryonic antigen radioimmunodetection and computed tomography for predicting the resectability of recurrent colorectal cancer. Ann Surg. 1997 Nov;226(5):621-31. [Article]
Drug created at June 13, 2005 13:24 / Updated at April 09, 2024 18:11


