Explore a selection of our essential drug information below, or:
Identification
- Summary
Vedolizumab is an integrin blocker and anti-inflammatory agent used to manage ulcerative colitis and Crohn's disease in adults.
- Brand Names
- Entyvio
- Generic Name
- Vedolizumab
- DrugBank Accession Number
- DB09033
- Background
Vedolizumab is a recombinant humanized IgG1 monoclonal antibody directed against the human lymphocyte α4β7 integrin, a key mediator of gastrointestinal inflammation implicated in diseases like ulcerative colitis or Crohn's disease.4 α4β7 integrin facilitates the interaction between lymphocytes and gut endothelial cells through the α4β7 integrin-MAdCAM1 interaction, leading to the mobilization of lymphocytes and thus contributing to gastrointestinal inflammation.4 Integrins implicated in cell migration into the intestinal tract included α2β2, α4β1, and α4β7; however, the selective activity of vedolizumab against α4β7 integrin has been thought to contribute to its more favorable safety profile compared to its predecessor natalizumab, the first integrin receptor antagonist approved by the FDA.3 Vedolizumab is administered by IV infusion over a period of 30 minutes; after the first dose, it is given again at two and six weeks and then every 8 weeks thereafter.4
Vedolizumab was developed by Takeda and approved by the FDA under the brand name ENTYVIO for the maintenance therapy of moderately to severely active Ulcerative Colitis and Crohn’s Disease in April and September 2023, respectively.6,7
- Type
- Biotech
- Groups
- Approved
- Biologic Classification
- Protein Based Therapies
Monoclonal antibody (mAb) - Protein Structure
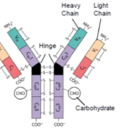
- Protein Chemical Formula
- C6528H10072N1732O2042S42
- Protein Average Weight
- 146837.0 Da
- Sequences
>Heavy Chain Sequence QVQLVQSGAEVKKPGASVKVSCKGSGYTFTSYWMHWVRQAPGQRLEWIGEIDPSESNTNY NQKFKGRVTLTVDISASTAYMELSSLRSEDTAVYYCARGGYDGWDYAIDYWGQGTLVTVS SASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQS SGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELAG APSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQY NSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRD ELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSR WQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
>Light Chain Sequence DVVMTQSPLSLPVTPGEPASISCRSSQSLAKSYGNTYLSWYLQKPGQSPQLLIYGISNRF SGVPDRFSGSGSGTDFTLKISRVEAEDVGVYYCLQGTHQPYTFGQGTKVEIKRTVAAPSV FIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSL SSTLTLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGEC
Download FASTA Format- Synonyms
- Vedolizumab
- External IDs
- LDP 02
- LDP-02
- LDP02
- MLN-0002
- MLN-02
- MLN0002
- MLN02
Pharmacology
- Indication
Vedolizumab is indicated for adult patients with moderately to severely active Ulcerative Colitis or Crohn’s disease.4
 Reduce drug development failure ratesBuild, train, & validate machine-learning modelswith evidence-based and structured datasets.Build, train, & validate predictive machine-learning models with structured datasets.
Reduce drug development failure ratesBuild, train, & validate machine-learning modelswith evidence-based and structured datasets.Build, train, & validate predictive machine-learning models with structured datasets.- Associated Conditions
Indication Type Indication Combined Product Details Approval Level Age Group Patient Characteristics Dose Form Treatment of Moderately to severely active crohn's disease •••••••••••• ••••••••• Treatment of Moderately to severely active ulcerative colitis •••••••••••• ••••••••• - Contraindications & Blackbox Warnings
 Prevent Adverse Drug Events TodayTap into our Clinical API for life-saving information on contraindications & blackbox warnings, population restrictions, harmful risks, & more.Avoid life-threatening adverse drug events with our Clinical API
Prevent Adverse Drug Events TodayTap into our Clinical API for life-saving information on contraindications & blackbox warnings, population restrictions, harmful risks, & more.Avoid life-threatening adverse drug events with our Clinical API- Pharmacodynamics
Non-clinical studies have shown that the pharmacodynamic effects of vedolizumab are reversible upon removal of the antibody: pharmacologic activity of cells inhibited by vedolizumab could be partially restored within 24 hours after removal, with near complete restoration within 4 days. There are no known drug interactions as vedolizumab is a humanized antibody and does not modulate the production of cytokines, which is known to affect drug metabolism.5
The α4β7 integrin is expressed on the surface of a discrete subset of memory T-lymphocytes that preferentially migrate into the gastrointestinal tract. Mucosal addressin cell adhesion molecule-1 (MAdCAM-1) is mainly expressed on gut endothelial cells and plays a critical role in homing T-lymphocytes to gut lymph tissue. The interaction of the α4β7 integrin with MAdCAM-1 has been implicated as an important contributor to chronic inflammation, a hallmark of ulcerative colitis and Crohn’s disease. Inhibition of α4β7 integrin by vedolizumab prevents the adhesion of lymphocytes to its natural ligand, thus decreasing the migration of memory T-lymphocytes across the endothelium into inflamed gastrointestinal parenchymal tissue.4
In clinical trials with vedolizumab at doses ranging from 0.2 to 10 mg/kg (which includes doses outside of the recommended dose), saturation of α4β7 receptors on subsets of circulating lymphocytes involved in gut-immune surveillance was observed.4
In clinical trials with vedolizumab at doses ranging from 0.2 to 10 mg/kg and 180 to 750 mg (which include doses outside of the recommended dose) in healthy subjects and in patients with ulcerative colitis or Crohn’s disease, vedolizumab did not elevate neutrophils, basophils, eosinophils, B-helper and cytotoxic T-lymphocytes, total memory helper T-lymphocytes, monocytes or natural killer cells.4
A reduction in gastrointestinal inflammation was observed in rectal biopsy specimens from Phase 2 ulcerative colitis patients exposed to vedolizumab for four or six weeks compared to placebo control as assessed by histopathology.4
In a study of 14 healthy subjects, vedolizumab did not affect the CD4+ lymphocyte cell counts, CD8+ lymphocyte cell counts, or the CD4+:CD8+ ratios in the CSF.4
- Mechanism of action
Vedolizumab is a humanized monoclonal antibody that specifically binds to the α4β7 integrin and blocks the interaction of α4β7 integrin with MAdCAM-1. Vedolizumab does not bind to or inhibit the function of the α4β1 and αEβ7 integrins and does not antagonize the interaction of α4 integrins with vascular cell adhesion molecule-1 (VCAM-1).4
Target Actions Organism AIntegrin alpha-4 antibodyHumans AIntegrin beta-7 antibodyHumans - Absorption
The intended route of administration is intravenous, therefore there is no absorption data and bioavailability is expected to be 100%. Following the administration of 300 mg of vedolizumab as a 30-minute intravenous infusion from week 0 to 2 and 300 mg every eight weeks starting from Week 6, the trough serum concentration of vedolizumab is 26.3 ± 12.9 and 27.4 ± 19.2 mcg/mL for Ulcerative Colitis and Crohn’s Disease patients respectively at week 6.5 At week 46, the trough serum concentration of vedolizumab is 11.2 ± 7.2 and 13.0 ± 9.1 mcg/mL for Ulcerative Colitis and Crohn’s Disease patients respectively.5
- Volume of distribution
Serum apparent volume of distribution at steady-state has been found to be moderately greater than the serum volume (approximately 5L).4,5 It is therefore expected to be confined to the systemic circulation, as expected for a high molecular weight protein.5
- Protein binding
Vedolizumab is a therapeutic monoclonal antibody and is not expected to bind to plasma proteins.5
- Metabolism
The expected consequence of metabolism is proteolytic degradation to small peptides and individual amino acids, and receptor-mediated clearance.5
- Route of elimination
Renal clearance is negligible as vedolizumab is a high molecular weight protein.
- Half-life
Vedolizumab has a long terminal elimination half-life of 25 days.4,5
- Clearance
Vedolizumab clearance depends on both linear and nonlinear pathways; the nonlinear clearance decreases with increasing concentrations. Population pharmacokinetic analyses indicated that the linear clearance was approximately 0.157 L/day or 0.180 to 0.266 ml/hr/kg.4,5
- Adverse Effects
 Improve decision support & research outcomesWith structured adverse effects data, including: blackbox warnings, adverse reactions, warning & precautions, & incidence rates. View sample adverse effects data in our new Data Library!Improve decision support & research outcomes with our structured adverse effects data.
Improve decision support & research outcomesWith structured adverse effects data, including: blackbox warnings, adverse reactions, warning & precautions, & incidence rates. View sample adverse effects data in our new Data Library!Improve decision support & research outcomes with our structured adverse effects data.- Toxicity
Elevated transaminase levels with or without elevated bilirubin have occurred in patients who have received this drug. Progressive multifocal leukoencephalopathy (PML) has not been reported with the use of this drug, however, it has occurred in patients who have received different integrin receptor antagonists and is therefore considered a risk for this product.4 The use of vedolizumab may increase the risk of developing infections, and one study found that nasopharyngitis occurs more frequently with vedolizumab than with THE placebo for Crohn’s disease patients.2
Available pharmacovigilance data, data from the ongoing pregnancy registry, and data from published case reports and cohort studies in pregnant women have not identified a vedolizumab-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. There are risks to the mother and the fetus associated with inflammatory bowel disease in pregnancy. No fetal harm was observed in animal reproduction studies with intravenous administration of vedolizumab to rabbits and monkeys at dose levels 20 times the recommended human dosage.4
Published data suggest that the risk of adverse pregnancy outcomes in women with inflammatory bowel disease (IBD) is associated with increased disease activity. Adverse pregnancy outcomes include preterm delivery (before 37 weeks of gestation), low birth weight (less than 2,500 g) infants, and small for gestational age at birth.4
Vedolizumab administered during pregnancy could affect immune responses in the in-utero-exposed newborn and infant. The clinical significance of low levels of vedolizumab in utero-exposed infants is unknown. The safety of administering live or live-attenuated vaccines in exposed infants is unknown.4
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of vedolizumab. Studies to evaluate the possible impairment of fertility or mutagenic potential of vedolizumab have not been performed.4
- Pathways
- Not Available
- Pharmacogenomic Effects/ADRs
- Not Available
Interactions
- Drug Interactions
- This information should not be interpreted without the help of a healthcare provider. If you believe you are experiencing an interaction, contact a healthcare provider immediately. The absence of an interaction does not necessarily mean no interactions exist.
Drug Interaction Integrate drug-drug
interactions in your softwareAbatacept The risk or severity of adverse effects can be increased when Abatacept is combined with Vedolizumab. Abciximab The risk or severity of adverse effects can be increased when Abciximab is combined with Vedolizumab. Adalimumab The risk or severity of infection can be increased when Adalimumab is combined with Vedolizumab. Adenovirus type 7 vaccine live The risk or severity of infection can be increased when Adenovirus type 7 vaccine live is combined with Vedolizumab. Aducanumab The risk or severity of adverse effects can be increased when Vedolizumab is combined with Aducanumab. - Food Interactions
- No interactions found.
Products
 Drug product information from 10+ global regionsOur datasets provide approved product information including:dosage, form, labeller, route of administration, and marketing period.Access drug product information from over 10 global regions.
Drug product information from 10+ global regionsOur datasets provide approved product information including:dosage, form, labeller, route of administration, and marketing period.Access drug product information from over 10 global regions.- Brand Name Prescription Products
Name Dosage Strength Route Labeller Marketing Start Marketing End Region Image Entyvio Injection, solution 108 mg Subcutaneous Takeda Pharma A/S 2021-02-10 Not applicable EU Entyvio Powder, for solution 300 mg / vial Intravenous Takeda Italia S.P.A. 2015-04-21 Not applicable Canada Entyvio Injection, solution 108 mg Subcutaneous Takeda Pharma A/S 2021-02-10 Not applicable EU Entyvio Injection, solution 108 mg/0.68mL Subcutaneous Takeda Pharma A/S 2023-09-27 Not applicable US Entyvio Injection, solution 108 mg Subcutaneous Takeda Pharma A/S 2021-02-10 Not applicable EU
Categories
- ATC Codes
- L04AG05 — Vedolizumab
- Drug Categories
- Amino Acids, Peptides, and Proteins
- Antibodies
- Antibodies, Monoclonal
- Antibodies, Monoclonal, Humanized
- Antineoplastic and Immunomodulating Agents
- Blood Proteins
- Cancer immunotherapy
- Gastrointestinal Agents
- Globulins
- Immunoglobulins
- Immunoproteins
- Immunosuppressive Agents
- Immunotherapy
- Integrin Receptor Antagonist
- Miscellaneous GI Drugs
- Proteins
- Selective Immunosuppressants
- Serum Globulins
- Chemical TaxonomyProvided by Classyfire
- Description
- Not Available
- Kingdom
- Organic Compounds
- Super Class
- Organic Acids
- Class
- Carboxylic Acids and Derivatives
- Sub Class
- Amino Acids, Peptides, and Analogues
- Direct Parent
- Peptides
- Alternative Parents
- Not Available
- Substituents
- Not Available
- Molecular Framework
- Not Available
- External Descriptors
- Not Available
- Affected organisms
- Not Available
Chemical Identifiers
- UNII
- 9RV78Q2002
- CAS number
- 943609-66-3
References
- General References
- Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER: The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009 Sep;330(3):864-75. doi: 10.1124/jpet.109.153973. Epub 2009 Jun 9. [Article]
- Wang MC, Zhang LY, Han W, Shao Y, Chen M, Ni R, Wang GN, Wei FX, Zhang YW, Xu XD, Zhang YC: PRISMA--efficacy and safety of vedolizumab for inflammatory bowel diseases: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2014 Dec;93(28):e326. doi: 10.1097/MD.0000000000000326. [Article]
- Cherry LN, Yunker NS, Lambert ER, Vaughan D, Lowe DK: Vedolizumab: an alpha4beta7 integrin antagonist for ulcerative colitis and Crohn's disease. Ther Adv Chronic Dis. 2015 Sep;6(5):224-33. doi: 10.1177/2040622315586970. [Article]
- FDA Approved Drug Products: ENTYVIO (vedolizumab) for injection, for intravenous use [Link]
- Assessment report: Entyvio (International non-proprietary name vedolizumab) [Link]
- Takeda Announces FDA Acceptance of BLA Resubmission for Investigational Subcutaneous Administration of Entyvio® (vedolizumab) for Maintenance Therapy in Moderately to Severely Active Ulcerative Colitis [Link]
- Takeda Announces FDA Acceptance of BLA for Subcutaneous Administration of ENTYVIO® (vedolizumab) for Maintenance Therapy in Moderately to Severely Active Crohn’s Disease [Link]
- External Links
- KEGG Drug
- D08083
- PubChem Substance
- 347910392
- 1538097
- ChEMBL
- CHEMBL1743087
- RxList
- RxList Drug Page
- Drugs.com
- Drugs.com Drug Page
- Wikipedia
- Vedolizumab
- FDA label
- Download (281 KB)
Clinical Trials
- Clinical Trials
Clinical Trial & Rare Diseases Add-on Data Package
Explore 4,000+ rare diseases, orphan drugs & condition pairs, clinical trial why stopped data, & more. Preview package Phase Status Purpose Conditions Count Start Date Why Stopped 100+ additional columns Unlock 175K+ rows when you subscribe.View sample dataNot Available Active Not Recruiting Not Available Crohn's Disease (CD) 1 somestatus stop reason just information to hide Not Available Active Not Recruiting Not Available Inflammatory Bowel Diseases (IBD) 1 somestatus stop reason just information to hide Not Available Active Not Recruiting Not Available Ulcerative Colitis 1 somestatus stop reason just information to hide Not Available Completed Not Available Crohn's Disease (CD) / Ulcerative Colitis 1 somestatus stop reason just information to hide Not Available Completed Not Available Inflammatory Bowel Diseases (IBD) 2 somestatus stop reason just information to hide
Pharmacoeconomics
- Manufacturers
- Not Available
- Packagers
- Not Available
- Dosage Forms
Form Route Strength Injection, powder, for solution Intravenous 300 MG/5ML Injection, powder, for solution Intravenous 33100000 mg Injection, powder, for solution Intravenous; Parenteral 300 MG Injection, powder, lyophilized, for solution Intravenous 300 mg/5mL Injection, solution Subcutaneous 108 MG Powder, for solution Intravenous 300 mg / vial Solution Intravenous 300.000 mg Solution Subcutaneous 108 mg / 0.68 mL Powder Intravenous 300 mg Injection, powder, lyophilized, for solution Intravenous Injection, solution Subcutaneous 108 mg/0.68mL Injection, powder, for solution Intravenous 300 mg Solution Subcutaneous 108.00 mg Solution Subcutaneous 10800000 mg Solution Subcutaneous 108 mg Injection, solution, concentrate Intravenous 300 mg/1vial - Prices
- Not Available
- Patents
Patent Number Pediatric Extension Approved Expires (estimated) Region US2012151248 No 2012-05-02 2032-05-02 US
Properties
- State
- Solid
- Experimental Properties
Property Value Source isoelectric point 7.6-8.3 European Medicines Agency Assessment Report (Procedure No.: EMEA/H/C/002782/0000)
Targets

- Kind
- Protein
- Organism
- Humans
- Pharmacological action
- Yes
- Actions
- Antibody
- General Function
- Integrins alpha-4/beta-1 (VLA-4) and alpha-4/beta-7 are receptors for fibronectin. They recognize one or more domains within the alternatively spliced CS-1 and CS-5 regions of fibronectin. They are also receptors for VCAM1. Integrin alpha-4/beta-1 recognizes the sequence Q-I-D-S in VCAM1. Integrin alpha-4/beta-7 is also a receptor for MADCAM1. It recognizes the sequence L-D-T in MADCAM1. On activated endothelial cells integrin VLA-4 triggers homotypic aggregation for most VLA-4-positive leukocyte cell lines. It may also participate in cytolytic T-cell interactions with target cells. ITGA4:ITGB1 binds to fractalkine (CX3CL1) and may act as its coreceptor in CX3CR1-dependent fractalkine signaling (PubMed:23125415). ITGA4:ITGB1 binds to PLA2G2A via a site (site 2) which is distinct from the classical ligand-binding site (site 1) and this induces integrin conformational changes and enhanced ligand binding to site 1 (PubMed:18635536, PubMed:25398877). Integrin ITGA4:ITGB1 represses PRKCA-mediated L-type voltage-gated channel Ca(2+) influx and ROCK-mediated calcium sensitivity in vascular smooth muscle cells via its interaction with SVEP1, thereby inhibiting vasocontraction (PubMed:35802072)
- Specific Function
- cell adhesion molecule binding
- Gene Name
- ITGA4
- Uniprot ID
- P13612
- Uniprot Name
- Integrin alpha-4
- Molecular Weight
- 114898.745 Da
References
- Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER: The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009 Sep;330(3):864-75. doi: 10.1124/jpet.109.153973. Epub 2009 Jun 9. [Article]
- FDA Approved Drug Products: ENTYVIO (vedolizumab) for injection, for intravenous use [Link]
- Kind
- Protein
- Organism
- Humans
- Pharmacological action
- Yes
- Actions
- Antibody
- General Function
- Integrin ITGA4/ITGB7 (alpha-4/beta-7) (Peyer patches-specific homing receptor LPAM-1) is an adhesion molecule that mediates lymphocyte migration and homing to gut-associated lymphoid tissue (GALT) (Probable). Integrin ITGA4/ITGB7 interacts with the cell surface adhesion molecules MADCAM1 which is normally expressed by the vascular endothelium of the gastrointestinal tract (PubMed:10837471, PubMed:14608374). Interacts also with VCAM1 and fibronectin, an extracellular matrix component (Probable). It recognizes one or more domains within the alternatively spliced CS-1 region of fibronectin (Probable). Interactions involve the tripeptide L-D-T in MADCAM1, and L-D-V in fibronectin (Probable). Integrin ITGAE/ITGB7 (alpha-E/beta-7, HML-1) is a receptor for E-cadherin (PubMed:10837471)
- Specific Function
- cell adhesion molecule binding
- Gene Name
- ITGB7
- Uniprot ID
- P26010
- Uniprot Name
- Integrin beta-7
- Molecular Weight
- 86902.415 Da
References
- Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER: The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009 Sep;330(3):864-75. doi: 10.1124/jpet.109.153973. Epub 2009 Jun 9. [Article]
- FDA Approved Drug Products: ENTYVIO (vedolizumab) for injection, for intravenous use [Link]
Drug created at February 20, 2015 22:30 / Updated at November 24, 2023 04:34


