Identification
- Summary
Salmon calcitonin is a synthetic peptide form of calcitonin used to inhibit bone resorption in the treatment of hypercalcemia, osteoporosis, and Paget's disease.
- Brand Names
- Calcimar, Fortical, Miacalcin
- Generic Name
- Salmon calcitonin
- DrugBank Accession Number
- DB00017
- Background
Synthetic peptide, 32 residues long formulated as a nasal spray.
- Type
- Biotech
- Groups
- Approved, Investigational
- Biologic Classification
- Protein Based Therapies
Hormones - Protein Structure
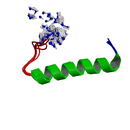
- Protein Chemical Formula
- C145H240N44O48S2
- Protein Average Weight
- 3431.853 Da
- Sequences
>DB00017 sequence CSNLSTCVLGKLSQELHKLQTYPRTNTGSGTP
Download FASTA Format- Synonyms
- Calcitonin (Salmon Synthetic)
- Calcitonin salmon
- Calcitonin salmon recombinant
- Calcitonin-salmon
- Calcitonin, salmon
- Calcitonina salmón sintética
- Recombinant salmon calcitonin
- Salmon calcitonin
- External IDs
- SMC-021
- SMC021
Pharmacology
- Indication
Used in the treatment of symptomatic Paget's disease for patients unresponsive to alternate treatments or intolerant to such treatments. In addition, it is used in emergency situations when serum calcium levels must be decreased quickly until the underlying condition is identified. It can also be added to existing therapeutic regimens for hypercalcemia such as intravenous fluids and furosemide, oral phosphate or corticosteroids, or other agents. Calcitonin can be used in patients with azotemia and cases where intravenous fluids would be contraindicated due to limited cardiac reserves. Also for the treatment of post-menopausal osteoporosis in women more than 5 years post-menopause.
 Reduce drug development failure ratesBuild, train, & validate machine-learning modelswith evidence-based and structured datasets.Build, train, & validate predictive machine-learning models with structured datasets.
Reduce drug development failure ratesBuild, train, & validate machine-learning modelswith evidence-based and structured datasets.Build, train, & validate predictive machine-learning models with structured datasets.- Associated Conditions
Indication Type Indication Combined Product Details Approval Level Age Group Patient Characteristics Dose Form Treatment of Hypercalcemia •••••••••••• •••••••••• •••••••• Prevention of Osteoporosis •••••••••••• •••••••••••• •••••••••• •••••••••••••• ••••••••• Management of Osteoporosis •••••••••••• ••••••••••••••• ••••• Management of Paget’s disease •••••••••••• •••••••••• ••••••••••• ••••••••• ••••••• ••••••••• Management of Symptomatic paget's disease •••••••••••• •••••••••• •••••••• - Contraindications & Blackbox Warnings
 Prevent Adverse Drug Events TodayTap into our Clinical API for life-saving information on contraindications & blackbox warnings, population restrictions, harmful risks, & more.Avoid life-threatening adverse drug events with our Clinical API
Prevent Adverse Drug Events TodayTap into our Clinical API for life-saving information on contraindications & blackbox warnings, population restrictions, harmful risks, & more.Avoid life-threatening adverse drug events with our Clinical API- Pharmacodynamics
Calcitonin inhibits bone resorption by osteoclasts (bone remodeling cells) and promotes bone formation by osteoblasts. This leads to a net increase in bone mass and a reduction in plasma calcium levels. It also promotes the renal excretion of ions such as calcium, phosphate, sodium, magnesium, and potassium by decreasing tubular reabsorption. In consequence, there is an increase in the jejunal secretion of water, sodium, potassium, and chloride.
- Mechanism of action
Calcitonin binds to the calcitonin receptor (found primarily in osteoclasts) which then enhances the production of vitamin D producing enzymes (25-hydroxyvitamine D-24-hydroxylase), leading to greater calcium retention and enhanced bone density. Binding of calcitonin to its receptor also activates adenylyl cyclase and the phosphatidyl-inositol-calcium pathway.
Target Actions Organism UCalcitonin receptor agonistHumans - Absorption
Salmon calcitonin is rapidly absorbed with bioavailability of 71% following subcutaneous injection and 66% following intramuscular injection in humans. Via the nasal route, the bioavailability varies between 3 to 5% relative to IM.
- Volume of distribution
0.15 to 0.3 L/kg
- Protein binding
Protein binding is about 30 to 40%.
- Metabolism
Salmon calcitonin primarily undergoes degradation in the kidneys to form pharmacologically inactive metabolites. It is also metabolized in the blood and the peripheral tissue.
- Route of elimination
Urine. Studies with injectable calcitonin show increases in the excretion of filtered phosphate, calcium, and sodium by decreasing their tubular reabsorption in the kidney.
- Half-life
Half-life elimination (terminal): I.M. 58 minutes; SubQ 59 to 64 minutes; Nasal: ~18 to 23 minutes
- Clearance
Not Available
- Adverse Effects
 Improve decision support & research outcomesWith structured adverse effects data, including: blackbox warnings, adverse reactions, warning & precautions, & incidence rates. View sample adverse effects data in our new Data Library!Improve decision support & research outcomes with our structured adverse effects data.
Improve decision support & research outcomesWith structured adverse effects data, including: blackbox warnings, adverse reactions, warning & precautions, & incidence rates. View sample adverse effects data in our new Data Library!Improve decision support & research outcomes with our structured adverse effects data.- Toxicity
Salmon calcitonin was shown to inhibit lactation in animals and is not recommend in nursing mothers. While research in animals have shown a decrease in fetal weight, no studies have yet shown similar results in humans. It is recommended however to proceed carefully when administering salmon calcitonin to pregnant women and consider if the benefits outweigh the risks. Because of its protein nature, salmon calcitonin may provoke an allergy reaction (bronchospams and swelling of the tongue/throat) that can turn into a full-blown anaphylactic response. The manufacturer also reports an increase in the risk of malignancies from oral route (0.7%) to intranasal route (2.4%) compared to placebo. The same may apply to IV, IM and SC routes since the systemic exposure is higher in those cases. Nausea is noticeable in some patients but tends to decrease with continued administration. Rhinitis, headaches and back pain have also been reported among others.
- Pathways
- Not Available
- Pharmacogenomic Effects/ADRs
- Not Available
Interactions
- Drug Interactions
- This information should not be interpreted without the help of a healthcare provider. If you believe you are experiencing an interaction, contact a healthcare provider immediately. The absence of an interaction does not necessarily mean no interactions exist.
Drug Interaction Integrate drug-drug
interactions in your softwareAbacavir Salmon calcitonin may decrease the excretion rate of Abacavir which could result in a higher serum level. Acalabrutinib The therapeutic efficacy of Salmon calcitonin can be decreased when used in combination with Acalabrutinib. Acebutolol The risk or severity of adverse effects can be increased when Acebutolol is combined with Salmon calcitonin. Aceclofenac Aceclofenac may decrease the excretion rate of Salmon calcitonin which could result in a higher serum level. Acemetacin Acemetacin may decrease the excretion rate of Salmon calcitonin which could result in a higher serum level. - Food Interactions
- Administer calcium supplement. Individuals who are taking salmon calcitonin for the treatment of postmenopausal osteoporosis should ingest at least 1000mg of elemental calcium daily (by food or through supplementation).
- Administer vitamin supplements. Individuals who are taking salmon calcitonin for the treatment of postmenopausal osteoporosis should take at least 400 international units of vitamin D daily.
Products
 Drug product information from 10+ global regionsOur datasets provide approved product information including:dosage, form, labeller, route of administration, and marketing period.Access drug product information from over 10 global regions.
Drug product information from 10+ global regionsOur datasets provide approved product information including:dosage, form, labeller, route of administration, and marketing period.Access drug product information from over 10 global regions.- International/Other Brands
- Calcimar
- Brand Name Prescription Products
Name Dosage Strength Route Labeller Marketing Start Marketing End Region Image Calcimar Solution 200 unit / mL Intramuscular; Subcutaneous Sanofi Aventis 1983-12-31 Not applicable Canada Calcitonin (salmon) Injection, BP Solution 200 unit / mL Intramuscular; Subcutaneous Sterimax Inc Not applicable Not applicable Canada Caltine Inj 100 Unit/ml (1ml Amp) Liquid 100 unit / mL Intramuscular; Subcutaneous Ferring Pharmaceuticals 1993-12-31 2014-07-25 Canada Caltine Inj 100unit/ml (0.5ml Amp) Liquid 100 unit / mL Intramuscular; Subcutaneous Ferring Pharmaceuticals 1993-12-31 2002-01-30 Canada Forcaltonin Injection, solution 100 IU Intramuscular; Intravenous; Subcutaneous Unigene Laboratories Inc. 2016-09-08 2008-11-20 EU - Generic Prescription Products
Name Dosage Strength Route Labeller Marketing Start Marketing End Region Image Apo-calcitonin Injectable Liquid 200 unit / mL Intramuscular; Subcutaneous Apotex Corporation 2003-05-01 2013-08-02 Canada Apo-calcitonin Nasal Spray Solution 200 unit / spray Nasal Apotex Corporation 2003-09-12 2013-10-01 Canada Calcitonin Salmon Spray, metered 200 [iU]/0.09mL Nasal Par Pharmaceutical, Inc. 2009-06-08 Not applicable US Calcitonin Salmon Spray, metered 200 [iU]/1 Nasal bryant ranch prepack 2008-12-09 Not applicable US Calcitonin Salmon Spray, metered 200 [iU]/0.09mL Nasal Physicians Total Care, Inc. 2011-12-09 Not applicable US
Categories
- ATC Codes
- H05BA01 — Calcitonin (salmon synthetic)
- Drug Categories
- Amino Acids, Peptides, and Proteins
- Bone Density Conservation Agents
- Bone Density, drug effects
- Calcitonin Preparations
- Calcium Homeostasis
- Calcium-Regulating Hormones and Agents
- Drugs that are Mainly Renally Excreted
- Hormones
- Hormones, Hormone Substitutes, and Hormone Antagonists
- Nerve Tissue Proteins
- Neuropeptides
- Parathyroid and Antiparathyroid Agents
- Parathyroid Hormones and Analogues
- Peptide Hormones
- Peptides
- Proteins
- Systemic Hormonal Preparations, Excl. Sex Hormones and Insulins
- Thyroid Products
- Chemical TaxonomyProvided by Classyfire
- Description
- Not Available
- Kingdom
- Organic Compounds
- Super Class
- Organic Acids
- Class
- Carboxylic Acids and Derivatives
- Sub Class
- Amino Acids, Peptides, and Analogues
- Direct Parent
- Peptides
- Alternative Parents
- Not Available
- Substituents
- Not Available
- Molecular Framework
- Not Available
- External Descriptors
- Not Available
- Affected organisms
- Humans and other mammals
Chemical Identifiers
- UNII
- 7SFC6U2VI5
- CAS number
- 47931-85-1
References
- Synthesis Reference
Marcos C. Poblet, Berta P. Obiols, Gemma J. Farres, "Procedure for preparing salmon calcitonin." U.S. Patent US5527881, issued October, 1991.
US5527881- General References
- Not Available
- External Links
- UniProt
- P01263
- Genbank
- Y00765
- KEGG Drug
- D00249
- KEGG Compound
- C06865
- PubChem Substance
- 46506061
- 36118
- Therapeutic Targets Database
- DAP001302
- PharmGKB
- PA448715
- RxList
- RxList Drug Page
- Wikipedia
- Calcitonin
Clinical Trials
- Clinical Trials
Phase Status Purpose Conditions Count 4 Completed Prevention Analgesia 1 4 Completed Treatment Fracture Forearm 1 4 Completed Treatment Hypothyroidism 1 4 Completed Treatment Pelvic Ring Fractures 1 4 Terminated Treatment Pain / Rib Fractures 1
Pharmacoeconomics
- Manufacturers
- Sanofi aventis us llc
- Astrazeneca lp
- Novartis pharmaceuticals corp
- Apotex inc
- Par pharmaceutical inc
- Upsher smith laboratories inc
- Packagers
- Apotex Inc.
- Novartis AG
- Par Pharmaceuticals
- Sandoz
- Upsher Smith Laboratories
- Dosage Forms
Form Route Strength Liquid Intramuscular; Subcutaneous 200 unit / mL Solution Nasal 200 unit / spray Solution Intramuscular; Subcutaneous 200 unit / mL Injection, solution Parenteral 100 I.E./ml Injection, solution Parenteral 50 I.E./ml Injection, solution Injection, solution Intramuscular; Subcutaneous 200 [iU]/1mL Powder Not applicable 1 g/1g Spray, metered Nasal 200 [iU]/0.09mL Injection, solution Intramuscular; Intravenous; Subcutaneous 100 IU/ML Injection, solution Intramuscular; Intravenous; Subcutaneous 50 IU/ML Spray Nasal 100 U.I. Spray Nasal 50 U.I. Solution 100 IU/1ml Solution 50 IU/1ml Liquid Intramuscular; Subcutaneous 100 unit / mL Injection, solution Intramuscular; Intravenous; Subcutaneous 100 IU Spray, metered Nasal 2200 [iU]/1mL Injection Intramuscular; Subcutaneous Spray Nasal Injection, solution 100 IU/1ml Injection, solution 50 IU/1ml Injection, solution Intramuscular; Subcutaneous 100 iu Injection Intramuscular; Subcutaneous 50 IU/ml Injection Intramuscular; Intravenous; Subcutaneous 100 iu/ml Injection Intramuscular; Intravenous; Subcutaneous 50 iu/ml Spray Nasal 200 iu/dose Spray, metered Nasal Injection, solution Intramuscular 200 [iU]/1mL Injection, solution Intramuscular; Subcutaneous 200 [USP'U]/1mL Injection, solution Intramuscular; Subcutaneous 200.0 [iU]/1.0mL Spray, metered Nasal 200 [iU]/1 Liquid Intramuscular; Intravenous; Subcutaneous 200 unit / mL Spray Nasal 200 unit / act Spray Nasal 200 IU/1dose Liquid Nasal 200 unit / spray Spray Nasal 200 IU Spray Nasal 100 IU Solution; spray Nasal 200 IU/1dose - Prices
Unit description Cost Unit Miacalcin 200 unit/act Solution 3.7ml Bottle 139.39USD bottle Calcitonin (Salmon) 200 unit/act Solution 3.7ml Bottle 123.28USD bottle Miacalcin For Inj, 2 unit = 1 Box 2ml Vial 63.59USD vial Miacalcin 200 unit nasal spray 44.68USD ml Calcitonin-salmon 200 unit sp 39.51USD ml Fortical 200 unit nasal spray 34.3USD ml Miacalcin 200 unit/ml vial 30.73USD ml Calcimar 200 iu/ml 29.94USD ml Caltine 100 (100 Iu/Ml) 100 iu/ml 8.81USD ml DrugBank does not sell nor buy drugs. Pricing information is supplied for informational purposes only.- Patents
Patent Number Pediatric Extension Approved Expires (estimated) Region US5733569 No 1998-03-31 2015-03-31 US US6440392 No 2002-08-27 2021-02-02 US USRE43580 No 2012-08-14 2021-02-02 US USRE40812 No 2009-06-30 2021-02-02 US
Properties
- State
- Liquid
- Experimental Properties
Property Value Source hydrophobicity -0.537 Not Available isoelectric point 8.86 Not Available
Targets

- Kind
- Protein
- Organism
- Humans
- Pharmacological action
- Unknown
- Actions
- Agonist
- General Function
- Receptor activity
- Specific Function
- This is a receptor for calcitonin. The activity of this receptor is mediated by G proteins which activate adenylyl cyclase. The calcitonin receptor is thought to couple to the heterotrimeric guanos...
- Gene Name
- CALCR
- Uniprot ID
- P30988
- Uniprot Name
- Calcitonin receptor
- Molecular Weight
- 59351.865 Da
References
- Bouizar Z, Fouchereau-Peron M, Taboulet J, Moukhtar MS, Milhaud G: Purification and characterization of calcitonin receptors in rat kidney membranes by covalent cross-linking techniques. Eur J Biochem. 1986 Feb 17;155(1):141-7. [Article]
- Stroop SD, Moore EE: Intracellular calcium increases mediated by a recombinant human calcitonin receptor. J Bone Miner Res. 1995 Apr;10(4):524-32. [Article]
- Sarkar A, Dickerson IM: Cloning, characterization, and expression of a calcitonin receptor from guinea pig brain. J Neurochem. 1997 Aug;69(2):455-64. [Article]
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [Article]
Drug created at June 13, 2005 13:24 / Updated at April 26, 2024 04:06


